| Yellowed strontium |
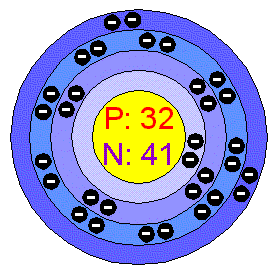
Discovered by A. Crawford of Scotland in 1790, strontium is the 38th element on the chart. In 1808, a scientist by the name 'Davy' isolated strontium for the first time using electrolysis. Strontium, as it was discovered in Strontian, Scotland, was named strontium as a memorial to the town that founded it.
Strontium is an element softer than calcium, and it decomposes faster in water. Very finely divided metal strontium is very dangerous and ignites spontaneously in open air. It is a silver metal, but it can change quickly when oxidized to a yellowish color. Since it ignites so openly, it is often stored under kerosene.
 Quick Fact: Strontium salt colors flames a deep red, and is often used in fireworks and flares.
Quick Fact: Strontium salt colors flames a deep red, and is often used in fireworks and flares.Strontium is used in Systems for Nuclear Auxilliary Power (SNAP) devices, and also in producind glass for color TV picture tubes. It produces ferrite magnets, and also helps to refine zinc.